This week’s lab began with our group finishing the plating of our twenty-six strains on optimal media containing starch. This test will be used to further characterize our strains. The next task involved scoring the growth of our twenty-six strains in liquid culture media. Our group then preformed colony counts of the irradiated Little Red Hill soil sample. The decrease in number of colonies with increase of dilution demonstrated good dilution technique.


 The plates also showed great diversity across the media we used. This maybe the result of the media facilitating the growth of certain organisms while inhibiting the growth of other organisms.
The plates also showed great diversity across the media we used. This maybe the result of the media facilitating the growth of certain organisms while inhibiting the growth of other organisms. 



Our group took 10 micro liters from each of the irradiated soil dilutions and plated them on new MA, PCA, 1/10 PCA, and 1/100 PCA media. Our group then selected 10 suspected organisms from the irradiated plates and plating them on new media. We parafilmed the plates and placed them in the incubator. From the results of the colony counts we determined the cfu/g for our irradiated soil samples.
THERE WAS A NICE EXCEL TABLE BUT THIS BLOG CAN COPE WITH TABLES.... the tables are at AirSet
We determined the percentage of survival by comparing the cfu/g of the irradiated soil counts over the cfu/g of the non-irradiate d soil counts.
THERE WAS A NICE EXCEL TABLE BUT THIS BLOG CAN COPE WITH TABLES.... the tables are at AirSet
It can be noted that the radiation significantly reduced the number of organisms in all cases with the exception of one of the Gobi samples. This lead to a class discussion of some of the possible reasons why the survivorship increased in the irradiated sample.
Our second lab day began with scoring the temperature variation plates of our twenty-six strains (10, 25, 30, 37, and 42 degrees Celsius) for growth and recording the data into our master chart. It should be noted due to a malfunction of the refrigerator, our 10 degree Celsius results may have been altered. It is also noted that 1/10 PCA media was contaminated, so three of our samples were bad. We then scored the growth of the twenty six strains, compared to the 25 degree Celsius plates, on media of varying NaCl concentrations (1, 3, 6, and 9%) and recorded that data into our master chart. For some reason our group was missing our strain 39 NaCl plates. There was significant variation of growth and diversity between the various temperatures and NaCl concentrations. This may be due to the fact that those variations inhibited or simulated the growth of certain organisms. Our group then streaked the twenty-six strains from the 25 degree Celsius plates to new optimal media to use as our new stock culture plates. For the samples that were contaminated we used our original stock vials to make new stock culture plates. Our class for the week ended with a group discussion of the upcoming midterm.
Our second lab day began with scoring the temperature variation plates of our twenty-six strains (10, 25, 30, 37, and 42 degrees Celsius) for growth and recording the data into our master chart. It should be noted due to a malfunction of the refrigerator, our 10 degree Celsius results may have been altered. It is also noted that 1/10 PCA media was contaminated, so three of our samples were bad. We then scored the growth of the twenty six strains, compared to the 25 degree Celsius plates, on media of varying NaCl concentrations (1, 3, 6, and 9%) and recorded that data into our master chart. For some reason our group was missing our strain 39 NaCl plates. There was significant variation of growth and diversity between the various temperatures and NaCl concentrations. This may be due to the fact that those variations inhibited or simulated the growth of certain organisms. Our group then streaked the twenty-six strains from the 25 degree Celsius plates to new optimal media to use as our new stock culture plates. For the samples that were contaminated we used our original stock vials to make new stock culture plates. Our class for the week ended with a group discussion of the upcoming midterm.




 LRH - growth on 1/100 PCA after irradiation:
LRH - growth on 1/100 PCA after irradiation: LRH - growth on PCA after irradiation:
LRH - growth on PCA after irradiation: LRH - growth on MA after irradiation:
LRH - growth on MA after irradiation:







 LRH -3 dilution tenth strength PCA:
LRH -3 dilution tenth strength PCA:


















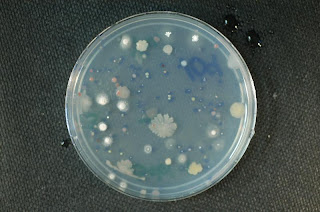
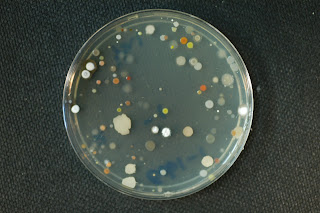



 Gobi-1 PCA:
Gobi-1 PCA: Gobi-1 1/10 strength PCA:
Gobi-1 1/10 strength PCA: Gobi-1 1/100 strength PCA:
Gobi-1 1/100 strength PCA:
